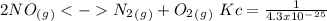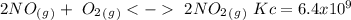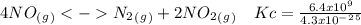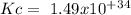Chemistry, 22.10.2019 21:00 kobiemajak
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g) + o2(g) ⇌ 2no(g) kc = 4.3 × 10−25 2no(g) + o2(g) ⇌ 2no2(g) kc = 6.4 × 109 determine the values of the equilibrium constants for the following equations at the same temperature: (a) 4no(g) ⇌ n2(g) + 2no2(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g)...
Questions


History, 11.11.2020 18:50


Mathematics, 11.11.2020 18:50

English, 11.11.2020 18:50



English, 11.11.2020 18:50




English, 11.11.2020 18:50




Biology, 11.11.2020 18:50

History, 11.11.2020 18:50



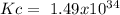
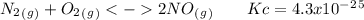
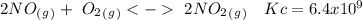
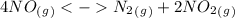
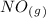 as a reactive in the target reaction and
as a reactive in the target reaction and