
A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burned in excess oxygen to yield 4.23 g of co2 and 1.01 g of h2o. another sample of the same compound, of mass 4.14 g, yielded 2.11 g of so3. a third sample, of mass 5.66 g, was burned under different conditions to yield 2.27 g of hno3 as the only nitrogen containing product. determine the empirical formula of the compound.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burn...
Questions








Computers and Technology, 10.09.2019 21:10




Mathematics, 10.09.2019 21:10





Mathematics, 10.09.2019 21:10


Social Studies, 10.09.2019 21:10

Spanish, 10.09.2019 21:10

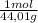 = 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt%
= 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt% 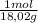 = 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%
= 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%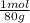 = 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%
= 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%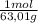 = 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%
= 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%

