

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
The ksp of agi is 8.3× 10–17. you titrate 25.00 ml of 0.08160 m nai with 0.05190 m agno3. calculate...
Questions


History, 18.03.2020 21:34


History, 18.03.2020 21:34






Mathematics, 18.03.2020 21:34

Mathematics, 18.03.2020 21:34

Social Studies, 18.03.2020 21:34




Biology, 18.03.2020 21:34

Biology, 18.03.2020 21:34

History, 18.03.2020 21:34


![K_{\text{sp}} = {\text{[Ag$^{+}$][I$^{-}$]} = 8.3\times 10^{-17}](/tpl/images/0339/7195/e8f96.png)
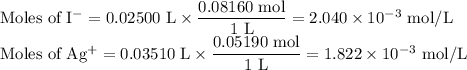
![\text{[I$^{-}$]} = \dfrac{0.218 \times 10^{-3}\text{ mol}}{\text{0.0610 L}} = 3.57 \times 10^{-3}\text{ mol/L}\\](/tpl/images/0339/7195/7cad1.png)
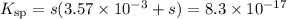
![\dfrac{3.57 \times 10^{-3}}{8.3\times 10^{-17}} = 4.3 \times 10^{13} 400\\\\\therefore s \ll 3.63 \times 10^{-3}\\K_{\text{sp}} = s\times 3.63 \times 10^{-3}= 8.3\times 10^{-17}\\\\s = \text{[Ag$^{+}$]} = \dfrac{8.3\times 10^{-17}}{3.63 \times 10^{-3}} =2.29 \times 10^{-14}\\\\\text{pAg} = -\log \left (2.29\times 10^{-14} \right) = \mathbf{13.64}](/tpl/images/0339/7195/167fe.png)

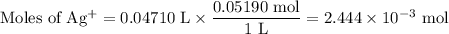
![\text{[Ag$^{+}$]} = \dfrac{0.404 \times 10^{-3}\text{ mol}}{\text{0.0721 L}} = 5.61 \times 10^{-3}\text{ mol/L}\\\text{pAg} = -\log(5.61 \times 10^{-3}) = \mathbf{2.25}](/tpl/images/0339/7195/d581c.png)


