
Chemistry, 21.10.2019 23:30 rachelsweeney10
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then adds iron powder to the conical flask at once. then, she stirs it briskly to initiate and facilitate the reaction between copper sulfate and iron powder. while stirring continuously, vanessa observes that the blue color of copper sulfate solution is fading substantially. moreover, she also observes the deposition of a reddish-brown mass at the bottom of the flask.
finally, a stage is reached where the solution becomes pale green in color. on further stirring of the solution, there is no change in the intensity of color. a distinct reddish-brown layer is deposited in the conical flask. however, on weighing the reaction mixture, she finds that the mass of the reaction mixture remains unchanged at 215.4 g. the reddish-brown layer is then separated from the pale green solution and allowed to dry in an oven. the moisture is completely evaporated, and the dried layer is weighed. its weight is found to be 63.5 g.
what is the mass of required iron powder and the formed iron sulfate in the chemical reaction?
a
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
b
the mass of iron powder required to complete the reaction was 63.5 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
c
the mass of iron powder required to complete the reaction was 159.6 g, while the mass of iron sulfate formed in the reaction was 63.5 g.
d
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 159.6 g.
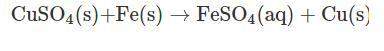

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then...
Questions

English, 31.01.2020 05:52

Mathematics, 31.01.2020 05:52

Mathematics, 31.01.2020 05:52

History, 31.01.2020 05:52

Mathematics, 31.01.2020 05:52


History, 31.01.2020 05:52

Mathematics, 31.01.2020 05:52

History, 31.01.2020 05:52


Mathematics, 31.01.2020 05:52


Social Studies, 31.01.2020 05:52



History, 31.01.2020 05:52

Mathematics, 31.01.2020 05:52

Physics, 31.01.2020 05:52




