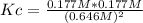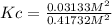Chemistry, 21.10.2019 18:10 lejeanjamespete1
Consider the reaction: 2hi(g) ⇄ h2(g) + i2(g). it is found that, when equilibrium is reached at a certain temperature, hi is 35.4 percent dissociated. calculate the equilibrium constant kc for the reaction at this temperature?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
Consider the reaction: 2hi(g) ⇄ h2(g) + i2(g). it is found that, when equilibrium is reached at a c...
Questions

Mathematics, 11.04.2021 15:10

Biology, 11.04.2021 15:10


Mathematics, 11.04.2021 15:20

Mathematics, 11.04.2021 15:20

English, 11.04.2021 15:20



Law, 11.04.2021 15:20

Social Studies, 11.04.2021 15:20


Mathematics, 11.04.2021 15:20

Social Studies, 11.04.2021 15:20


English, 11.04.2021 15:30

Health, 11.04.2021 15:30

Mathematics, 11.04.2021 15:30


Biology, 11.04.2021 15:30

![Kc = \frac{[H2]*[I2]}{[HI]^2}](/tpl/images/0338/7477/a79bd.png)