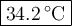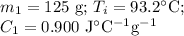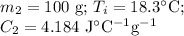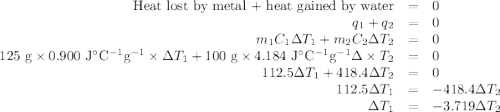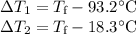Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
A125g metal block at a temperature of 93.2 degrees celsius was immersed in 100g of water at 18.3 deg...
Questions




Social Studies, 03.06.2021 03:20


Social Studies, 03.06.2021 03:20





Mathematics, 03.06.2021 03:30

Mathematics, 03.06.2021 03:30

History, 03.06.2021 03:30


Computers and Technology, 03.06.2021 03:30

Mathematics, 03.06.2021 03:30



Health, 03.06.2021 03:30

Mathematics, 03.06.2021 03:30