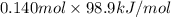Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1
You know the right answer?
Calculate the amount of energy in the form of heat that is produced when a volume of 3.43 l of so2(g...
Questions

Mathematics, 03.05.2021 19:20





Mathematics, 03.05.2021 19:20




English, 03.05.2021 19:20

Mathematics, 03.05.2021 19:20

Mathematics, 03.05.2021 19:20



Chemistry, 03.05.2021 19:20


Mathematics, 03.05.2021 19:20



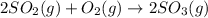
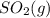 and
and 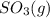 are as follows.
are as follows.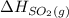 = -296.8 kJ/mol
= -296.8 kJ/mol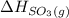 = -395.7 kJ/mol
= -395.7 kJ/mol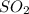 =
= 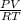
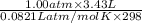 (as 1 bar = 1 atm (approximately))
(as 1 bar = 1 atm (approximately))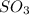 are also 0.140.
are also 0.140.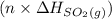 and
and 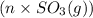
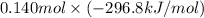 and
and 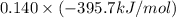 respectively.
respectively.![0.140 \times [-296.8 kJ/mol - (-395.7 kJ/mol)]](/tpl/images/0333/5672/320d5.png)