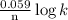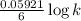Chemistry, 19.10.2019 05:10 bethdove9466
What is the value of the equilibrium constant for the reaction between cr(s) and cu^2+ (aq) at 25 degree c? cr(s) + cu^2+ (q)rightarrow cr^3+ (aq) + cu(s) if your calculator has overflow issues with this problem, you can use this identity to solve for k: if log(k) = x + y then k = 10^x times 10^y 3 times 10^109 -6 times 10^5 2 times 10^18 1 times 10^2 3 times 10^40

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
What is the value of the equilibrium constant for the reaction between cr(s) and cu^2+ (aq) at 25 de...
Questions

English, 28.11.2021 20:20







French, 28.11.2021 20:30

Chemistry, 28.11.2021 20:30

History, 28.11.2021 20:30


Mathematics, 28.11.2021 20:30

Mathematics, 28.11.2021 20:30

English, 28.11.2021 20:30


Computers and Technology, 28.11.2021 20:30

Mathematics, 28.11.2021 20:30


Chemistry, 28.11.2021 20:30

Mathematics, 28.11.2021 20:30