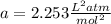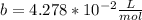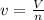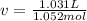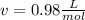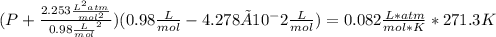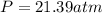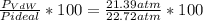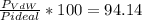1.according to the ideal gas law, a 1.052 mol sample of methane gas in a 1.031 l container at 271.3 k should exert a pressure of 22.72 atm. by what percent does the pressure calculated using the van der waals' equation differ from the ideal pressure? for ch4 gas,
a = 2.253 l2atm/mol2 and
b = 4.278×10-2 l/mol.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

Chemistry, 23.06.2019 10:30
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1
You know the right answer?
1.according to the ideal gas law, a 1.052 mol sample of methane gas in a 1.031 l container at 271.3...
Questions



English, 13.04.2021 20:40

Mathematics, 13.04.2021 20:40

Mathematics, 13.04.2021 20:40

Mathematics, 13.04.2021 20:40



Biology, 13.04.2021 20:40


Mathematics, 13.04.2021 20:40

English, 13.04.2021 20:40


Arts, 13.04.2021 20:40

Geography, 13.04.2021 20:40

Mathematics, 13.04.2021 20:40