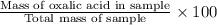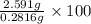Chemistry, 19.10.2019 00:30 reagriffis24
Oxalic acid, a diprotic acid having the formula h2c2o4, is used to clean the rust out of radiators in cars. a sample of an oxalic acid mixture was analyzed by titrating a 0.2816 g sample dissolved in water with 0.0461 m naoh. a volume of 11.49 ml of the base was required to completely neutralize the oxalic acid. what was the percentage by mass of oxalic acid in the sample?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3
You know the right answer?
Oxalic acid, a diprotic acid having the formula h2c2o4, is used to clean the rust out of radiators i...
Questions


History, 05.05.2020 20:26

English, 05.05.2020 20:26

Mathematics, 05.05.2020 20:26





Mathematics, 05.05.2020 20:26

Health, 05.05.2020 20:26

Mathematics, 05.05.2020 20:26

History, 05.05.2020 20:26


History, 05.05.2020 20:26

Biology, 05.05.2020 20:26



Mathematics, 05.05.2020 20:26


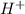 ions produced by
ions produced by 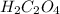 with total number of moles of
with total number of moles of 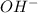 ions produced by NaOH.
ions produced by NaOH.
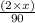 ........... (1) (We multiply by 2 because Oxalic Acid is a diprotic acid)
........... (1) (We multiply by 2 because Oxalic Acid is a diprotic acid)