
Chemistry, 19.10.2019 00:30 hannahbannana98
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthalein as the indicator. in the reaction of oxalic acid with naoh, every 1 mole of oxalic acid is deprotonated by 2 moles of naoh. the molecular weight of oxalic acid is 90.03 g/mol and you use 0.60 g in 50 ml water for the titration. you use 15.20 ml of your naoh solution in order to reach the endpoint of the titration. what is the concentration of your naoh solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthale...
Questions

Biology, 25.08.2019 22:10


Health, 25.08.2019 22:10

Mathematics, 25.08.2019 22:10

Social Studies, 25.08.2019 22:10

Mathematics, 25.08.2019 22:10

Health, 25.08.2019 22:10



Business, 25.08.2019 22:10

Mathematics, 25.08.2019 22:10


Social Studies, 25.08.2019 22:10

Mathematics, 25.08.2019 22:10


Biology, 25.08.2019 22:10

Mathematics, 25.08.2019 22:10

History, 25.08.2019 22:10


History, 25.08.2019 22:10

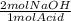 = 0.0133 mol NaOH
= 0.0133 mol NaOH

