Chemistry, 19.10.2019 00:10 jimperez9616
During world war ii, a portable source of hydrogen gas was needed for weather balloons, and solid metal hydrides were the most convenient form. many metal hydrides react with water to generate the metal hydroxide and hydrogen. two candidates were lithium hydride and magnesium hydride. what volume of gas is formed from 1.40 lb of each hydride at 750. torr and 27°c?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1


Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
During world war ii, a portable source of hydrogen gas was needed for weather balloons, and solid me...
Questions






Business, 07.11.2019 22:31





English, 07.11.2019 22:31


Computers and Technology, 07.11.2019 22:31







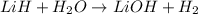
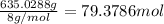
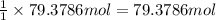 of hydrogen gas.
of hydrogen gas.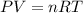
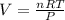
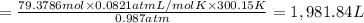
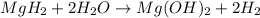
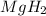 = 1.40 lb = 635.0288 g
= 1.40 lb = 635.0288 g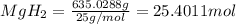
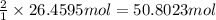 of hydrogen gas.
of hydrogen gas.