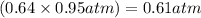Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
Amixture of he and ne at a total pressure of 0.95 atm is found to contain 0.32 mol of he and 0.56 mo...
Questions


Physics, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40


English, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40




Social Studies, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40




Mathematics, 20.11.2020 18:40


Health, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

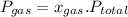
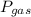 is partial pressure of a gas in mixture,
is partial pressure of a gas in mixture, 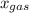 is mole fraction of a gas in mixture and
is mole fraction of a gas in mixture and 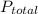 is total pressure of mixture.
is total pressure of mixture.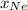 = (number of moles of Ne)/(Total number of moles in mixture)
= (number of moles of Ne)/(Total number of moles in mixture)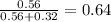
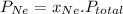 =
=