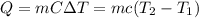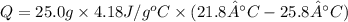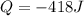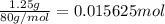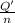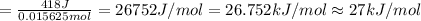Chemistry, 19.10.2019 00:00 tjacqueline9753
In order to measure the enthalpy change for this reaction, 1.25 g of nh4no3 is dissolved in enough water to make 25.0 ml of solution. the initial temperature is 25.8 degrees c and the final temperature (after the solid dissolves) is 21.9 degrees c. calculate the change in enthalpy for the reaction. (use 1.0g/ml as the density of the solution and 4.18 j/g . degrees c as the specific heat capacity.) express the answer to two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
In order to measure the enthalpy change for this reaction, 1.25 g of nh4no3 is dissolved in enough w...
Questions



Mathematics, 16.10.2020 03:01

Geography, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01

Social Studies, 16.10.2020 03:01



History, 16.10.2020 03:01

Chemistry, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01

History, 16.10.2020 03:01

History, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01


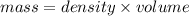
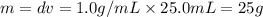
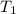 = 25.8°C
= 25.8°C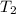 = 21.8°C
= 21.8°C