Chemistry, 18.10.2019 22:00 campbellkruger
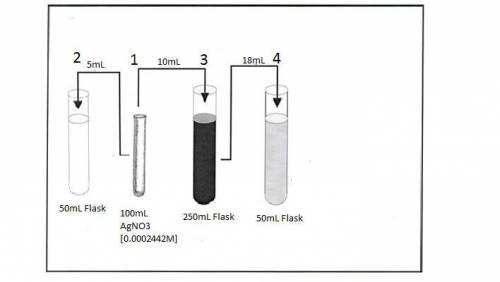
Astudent obtains 100 ml of a 0.0002442 m solution of agno 3 (silver nitrate). he labels this "solution #1". he then pipets 5 ml of solution #1 into a 50 ml volumetric flask and dilutes to the mark with water. he labels this "solution #2." he then pipets 10 ml of solution #1 into a 250 ml volumetric flask and dilutes to the mark with water. this is "solution #3". finally, the prepares "solution #4" by pipetting 18 ml of solution #3 into a 50 ml volumetric flask and dilutes to the mark with water. draw relational pictures to illustrate the relationship between the original solution and all of the diluted solutions that the student prepared. what is the concentration of silver nitrate in solution #4?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1

Chemistry, 23.06.2019 11:00
Which example is a mechanical wave? a.microwave b.radio wave c.water wave d.ultraviolet light
Answers: 1
You know the right answer?
Astudent obtains 100 ml of a 0.0002442 m solution of agno 3 (silver nitrate). he labels this "soluti...
Questions

Mathematics, 25.07.2019 09:30



Mathematics, 25.07.2019 09:30

Physics, 25.07.2019 09:30


Mathematics, 25.07.2019 09:30



History, 25.07.2019 09:30

English, 25.07.2019 09:30

Mathematics, 25.07.2019 09:30




History, 25.07.2019 09:30


Health, 25.07.2019 09:30

Mathematics, 25.07.2019 09:30

Arts, 25.07.2019 09:30