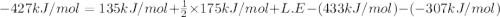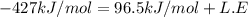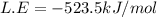Chemistry, 18.10.2019 19:20 ineedhelp2285
Consider an ionic compound, mx, composed of generic metal m and generic, gaseous halogen x. the enthalpy of formation of mxis δh∘f=−427kj/mol. the enthalpy of sublimation of mis δhsub=135kj/mol. the ionization energy of mis ie=433kj/mol. the electron affinity of xis δhea=−307kj/mol. (refer to the hint). the bond energy of x2is be=175kj/mol. determine the lattice energy of mx.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Consider an ionic compound, mx, composed of generic metal m and generic, gaseous halogen x. the enth...
Questions

History, 05.02.2021 18:50

Social Studies, 05.02.2021 18:50


Chemistry, 05.02.2021 18:50

Mathematics, 05.02.2021 18:50

Mathematics, 05.02.2021 18:50


Computers and Technology, 05.02.2021 18:50

Mathematics, 05.02.2021 18:50

Mathematics, 05.02.2021 18:50

Biology, 05.02.2021 18:50

Mathematics, 05.02.2021 18:50


English, 05.02.2021 18:50

Mathematics, 05.02.2021 18:50


Mathematics, 05.02.2021 18:50

Mathematics, 05.02.2021 18:50


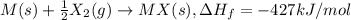 ....[1]
....[1]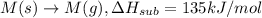 ....[2]
....[2]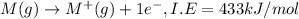 ....[3]
....[3]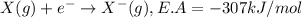 ....[4]
....[4]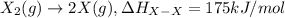 ....[5]
....[5]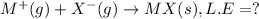 ....[6]
....[6]