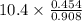Chemistry, 18.10.2019 19:10 Chrissyx4750
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in part a, and the temperature and volume were constant at values of 303 k and 2.00 l, respectively. if the pressure was 10.4 atm prior to the reaction, what would be the expected pressure after the reaction was completed?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3


Chemistry, 23.06.2019 06:40
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in par...
Questions


Mathematics, 21.01.2020 10:31

History, 21.01.2020 10:31





Mathematics, 21.01.2020 10:31


Mathematics, 21.01.2020 10:31

Mathematics, 21.01.2020 10:31

Mathematics, 21.01.2020 10:31

Biology, 21.01.2020 10:31





Biology, 21.01.2020 10:31


History, 21.01.2020 10:31

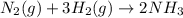
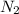 + moles of
+ moles of 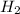 = 0.908 mol
= 0.908 mol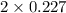 = 0.454 mol
= 0.454 mol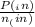 =
= 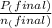
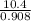 =
= 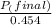
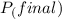 =
=