Chemistry, 18.10.2019 19:10 daverius3153
7. nh2co2nh4(s) when heated to 450 k undergoes the following reaction to produce a system which reaches equilibrium: nh2co2nh4(s) ⇀↽ 2 nh3(g) + co2(g) the total pressure in the closed container under these condition is found to be 0.843 atm. calculate a value for the equilibrium constant, kp. a) 0.00701 b) 0.0888 c) 0.222 d) 0.599

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
7. nh2co2nh4(s) when heated to 450 k undergoes the following reaction to produce a system which reac...
Questions













Mathematics, 20.02.2020 22:46

Biology, 20.02.2020 22:46



Mathematics, 20.02.2020 22:47


Mathematics, 20.02.2020 22:47

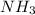 and
and 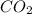 are gaseous. Hence equilibrium constant depends upon partial pressures of
are gaseous. Hence equilibrium constant depends upon partial pressures of 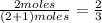
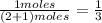
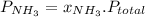 and P_{CO_{2}}= x_{CO_{2}}.P_{total}
and P_{CO_{2}}= x_{CO_{2}}.P_{total}