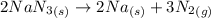Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. this causes sodium azide (nan3) to decompose explosively according to the following reaction: 2nan3 (s) → 2na(s) + 3n2(g) what mass of nan3 must be reacted to inflate an air bag to 70.0 l at stp?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1


Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
Air bags are activated when a severe impact causes a steel ball to compress a spring and electricall...
Questions

Mathematics, 28.01.2020 05:31




History, 28.01.2020 05:31


Mathematics, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31






Social Studies, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31


Biology, 28.01.2020 05:31



Biology, 28.01.2020 05:31

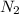 = 3.1214 moles
= 3.1214 moles