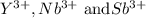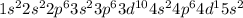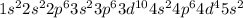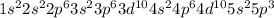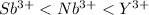Chemistry, 18.10.2019 01:30 tariqobrien6241
The actual cation radii are y3+ = 104 pm, nb3+ = 86 pm, and sb3+ = 90 pm. explain this trend. (hint: you should consider the electron configurations! ) [

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
The actual cation radii are y3+ = 104 pm, nb3+ = 86 pm, and sb3+ = 90 pm. explain this trend. (hint:...
Questions

Mathematics, 25.01.2021 19:40


Mathematics, 25.01.2021 19:40


Mathematics, 25.01.2021 19:40

Mathematics, 25.01.2021 19:40


Mathematics, 25.01.2021 19:40

Computers and Technology, 25.01.2021 19:40

Mathematics, 25.01.2021 19:40









Health, 25.01.2021 19:40