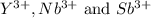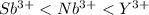Chemistry, 18.10.2019 01:30 untouchedyannaa
Given the following cations, list them in the expected order from smallest to largest: y3+, nb3+, sb3+

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Given the following cations, list them in the expected order from smallest to largest: y3+, nb3+, s...
Questions


Mathematics, 19.11.2020 07:40

Computers and Technology, 19.11.2020 07:40

Mathematics, 19.11.2020 07:40

Mathematics, 19.11.2020 07:40





Mathematics, 19.11.2020 07:40


History, 19.11.2020 07:40

Chemistry, 19.11.2020 07:40

World Languages, 19.11.2020 07:40

History, 19.11.2020 07:40




Mathematics, 19.11.2020 07:40