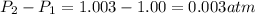Chemistry, 18.10.2019 01:30 Queenashley3232
At the normal melting point of nacl, 801 degrees c, its enthalpy of fusion is 28.8 kj / mol. the density of the solid is 2.165 g/cm^3 , and the density of the liquid is 1.733 g/ cm^3. what pressure increase is needed to raise the melting point by 1.00 degrees c?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
At the normal melting point of nacl, 801 degrees c, its enthalpy of fusion is 28.8 kj / mol. the den...
Questions

Biology, 26.09.2019 23:10

Biology, 26.09.2019 23:10




Mathematics, 26.09.2019 23:10


Biology, 26.09.2019 23:10

Mathematics, 26.09.2019 23:10


History, 26.09.2019 23:10


Mathematics, 26.09.2019 23:10







![\ln(\frac{P_2}{P_1})=\frac{\Delta H}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0330/0903/5c76e.png)
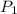 = initial pressure which is the pressure at normal boiling point = 1 atm
= initial pressure which is the pressure at normal boiling point = 1 atm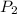 = final pressure = ?
= final pressure = ? = Enthalpy change of the reaction = 28.8 kJ/mol = 28800 J/mol (Conversion factor: 1 kJ = 1000 J)
= Enthalpy change of the reaction = 28.8 kJ/mol = 28800 J/mol (Conversion factor: 1 kJ = 1000 J)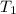 = initial temperature =
= initial temperature = ![801^oC=[801+273]K=1074K](/tpl/images/0330/0903/9b4bf.png)
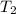 = final temperature =
= final temperature = ![(801+1.00)^oC=802.00=[802+273]K=1075K](/tpl/images/0330/0903/fe656.png)
![\ln(\frac{P_2}{1})=\frac{28800J/mol}{8.314J/mol.K}[\frac{1}{1074}-\frac{1}{1075}]\\\\\ln P_2=3\times 10^{-3}atm\\\\P_2=e^{3\times 10^{-3}}=1.003atm](/tpl/images/0330/0903/b8d88.png)