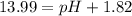Chemistry, 18.10.2019 01:30 DragonWarrior203
Determine the ph of the following base solutions. (assume that all solutions are at 25°c and the ion-product constant of water, kw, is 1.01 ✕ 10−14.) (a) 1.39 ✕ 10−2 m naoh webassign will check your answer for the correct number of significant figures. (b) 0.0051 m al(oh)3 webassign will check your answer for the correct number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
Determine the ph of the following base solutions. (assume that all solutions are at 25°c and the ion...
Questions















Mathematics, 31.08.2020 02:01




Computers and Technology, 31.08.2020 02:01

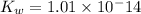
![K_w=[H^+][OH^-]](/tpl/images/0330/0905/bc68a.png)
![1.01\times 10^-{14}=[H^+][OH^-]](/tpl/images/0330/0905/ef4b3.png)
![-\log[1.01\times 10^-{14}]=(-\log [H^+])+(-\log [OH^-])](/tpl/images/0330/0905/fdeff.png)
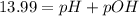
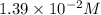 of NaOH.
of NaOH.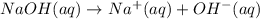
![[OH^-]=1\times [NaOH]=1\times 1.39\times 10^{-2} M=1.39\times 10^{-2} M](/tpl/images/0330/0905/52ef4.png)
![pOH=-\log[1.39\times 10^{-2} M]=1.86](/tpl/images/0330/0905/dac37.png)
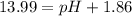
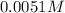 of NaOH.
of NaOH.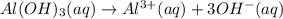
![[OH^-]=3\times [Al(OH)_3]=3\times 0.0051 M=0.0153 M](/tpl/images/0330/0905/db0a1.png)
![pOH=-\log[0.0153 M]=1.82](/tpl/images/0330/0905/47516.png)