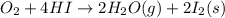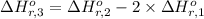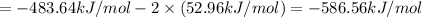Chemistry, 18.10.2019 03:30 miya257916
Calculate the standard enthalpy of the 3rd reaction using the given data: h2(g) +126) +2 hio) a, hº = +52.96 kj/mol 2 h2(g) + o2(g) + 2 h209) a, hº = - 483.64 kj/mol 4 hi) + o2(g) +2 12(8) + 2 h206) a, hº =? a. h=-589.5619/mol calculate the internal energy change for reaction (3) in the previous problem.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3


Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
You know the right answer?
Calculate the standard enthalpy of the 3rd reaction using the given data: h2(g) +126) +2 hio) a, hº...
Questions





Mathematics, 16.12.2020 20:50

Mathematics, 16.12.2020 20:50



Mathematics, 16.12.2020 20:50


Mathematics, 16.12.2020 20:50



Mathematics, 16.12.2020 20:50

English, 16.12.2020 20:50

Computers and Technology, 16.12.2020 20:50

English, 16.12.2020 20:50


Mathematics, 16.12.2020 20:50

 ...[1]
...[1]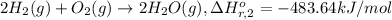 ...[2]
...[2]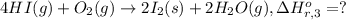 ..[3]
..[3]