Chemistry, 18.10.2019 03:30 GamerGirl15
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxide gas and liquid water. a. write the balanced equation for the reaction b. if there are 0.500 moles of copper metal present how many moles of nitric acid are required for the reaction? how many moles of nitrogen dioxide gas are formed?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1


Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxid...
Questions

History, 12.11.2020 06:20


Mathematics, 12.11.2020 06:20

History, 12.11.2020 06:20


Arts, 12.11.2020 06:20

Mathematics, 12.11.2020 06:20




Mathematics, 12.11.2020 06:20


Mathematics, 12.11.2020 06:20

Physics, 12.11.2020 06:20

English, 12.11.2020 06:20

Business, 12.11.2020 06:20

Social Studies, 12.11.2020 06:20



Biology, 12.11.2020 06:20

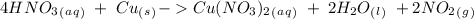
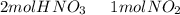
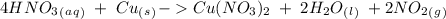
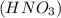 we have to use the molar ratio in the balence reaction:
we have to use the molar ratio in the balence reaction: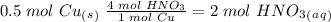
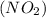 we have to follow the same logic:
we have to follow the same logic: