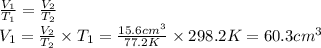Chemistry, 18.10.2019 00:30 lesliealvarado1022
Arubber balloon was filled with helium at 25.0˚c and placed in a beaker of liquid nitrogen at -196.0˚c. the volume of the cold helium was 15.6 cm3. assuming ideal gas behavior and isobaric conditions, what was the volume of the helium at 25.0˚c?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1


Chemistry, 23.06.2019 11:30
Bridget is in science class. her teacher gives her two unknown substances and asks her to determine their relative ph. she places a piece of red litmus paper into both substances. the litmus paper turns purple when she places it into substance i. the litmus paper turns blue when she places it into substance ii. a. substance i is a neutral substance and substance ii is an acid. b. substance i is a neutral substance and substance ii is a base. c. substance i is an acid and substance ii is a base. d. substance i is a base and substance ii is a neutral substance.
Answers: 1
You know the right answer?
Arubber balloon was filled with helium at 25.0˚c and placed in a beaker of liquid nitrogen at -196.0...
Questions



Physics, 28.11.2019 16:31

Social Studies, 28.11.2019 16:31


Chemistry, 28.11.2019 16:31


History, 28.11.2019 16:31



World Languages, 28.11.2019 16:31

Advanced Placement (AP), 28.11.2019 16:31

Mathematics, 28.11.2019 16:31

Social Studies, 28.11.2019 16:31

History, 28.11.2019 16:31

Mathematics, 28.11.2019 16:31