
Chemistry, 18.10.2019 01:00 19thomasar
The equilibrium constant kp for the reaction (ch3),cci (g) = (ch3),c=ch, (g) + hcl (g) is 3.45 at 500. k. (5.00 x 10k) calculate the value of kc at 500. k. for the same reaction, calculate the molar concentration of reactants and products at equilibrium if initially 1.00 mol of (ch3),cci was placed in a 5.00 l vessel.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

You know the right answer?
The equilibrium constant kp for the reaction (ch3),cci (g) = (ch3),c=ch, (g) + hcl (g) is 3.45 at 50...
Questions


Mathematics, 02.12.2021 20:00

English, 02.12.2021 20:00

Computers and Technology, 02.12.2021 20:00




Physics, 02.12.2021 20:00


Mathematics, 02.12.2021 20:00


Chemistry, 02.12.2021 20:00

Mathematics, 02.12.2021 20:00



Engineering, 02.12.2021 20:00


Mathematics, 02.12.2021 20:00

Spanish, 02.12.2021 20:00

 for the reaction is 6.32 and concentrations of
for the reaction is 6.32 and concentrations of 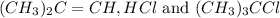 is 0.094 M, 0.094 M and 0.106 M respectively.
is 0.094 M, 0.094 M and 0.106 M respectively.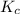 is given by the formula:
is given by the formula: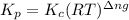
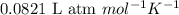
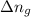 = change in number of moles of gas particles =
= change in number of moles of gas particles = 
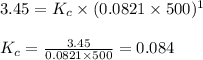
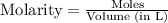
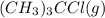 = 1.00 mol
= 1.00 mol
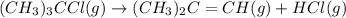
![K_c=\frac{[(CH_3)_2C=CH]\times [HCl]}{[(CH_3)_3CCl]}](/tpl/images/0329/9758/8a400.png)

![[(CH_3)_2C=CH]=x=0.094M](/tpl/images/0329/9758/5587a.png)
![[HCl]=x=0.094M](/tpl/images/0329/9758/e3b5a.png)
![[(CH_3)_3CCl]=(0.2-x)=(0.2-0.094)=0.106M](/tpl/images/0329/9758/364df.png)


