
Chemistry, 17.10.2019 19:00 unknown235
Which one of the following statements is true?
a. the enthalpy change for a reaction is independent of the state of the reactants and products.
b. enthalpy is an intensive property.
c. enthalpy is a state function.
d. h is the value of q measured under conditions of constant volume.
e. the enthalpy change of a reaction is the reciprocal of the δh of the reverse reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2

Chemistry, 23.06.2019 13:00
If volume remains the same while the mass of a substance the density of the substance
Answers: 1
You know the right answer?
Which one of the following statements is true?
a. the enthalpy change for a reaction is inde...
a. the enthalpy change for a reaction is inde...
Questions

Social Studies, 20.01.2020 11:31


English, 20.01.2020 11:31

History, 20.01.2020 11:31



Mathematics, 20.01.2020 11:31


Mathematics, 20.01.2020 11:31

Mathematics, 20.01.2020 11:31


Mathematics, 20.01.2020 11:31




History, 20.01.2020 11:31

English, 20.01.2020 11:31

Physics, 20.01.2020 11:31

Arts, 20.01.2020 11:31

Mathematics, 20.01.2020 11:31

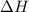 and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.
and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.

