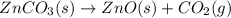The decomposition of znco3(s) into zno(s) and co2(g) at constant pressure requires the addition of 71.5 kj of heat per mole of znco3. you may want to reference (pages 177 - 178) section 5.4 while completing this problem. part apart complete write a balanced equation for the reaction. express your answer as a chemical equation. identify all of the phases in your answer. znco3(s)→zno(s)+co2(g) previous answers correct the reactant and products of the decomposition reaction are given in the introduction. znco3(s) is broken down into zno(s) and co2(g). part b what is the enthalpy of the reaction above?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1


Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
The decomposition of znco3(s) into zno(s) and co2(g) at constant pressure requires the addition of 7...
Questions


Mathematics, 08.07.2019 19:50


Chemistry, 08.07.2019 19:50


History, 08.07.2019 19:50

History, 08.07.2019 19:50


History, 08.07.2019 19:50

History, 08.07.2019 19:50



English, 08.07.2019 19:50

Mathematics, 08.07.2019 19:50

English, 08.07.2019 19:50


Geography, 08.07.2019 19:50

Geography, 08.07.2019 19:50

Geography, 08.07.2019 19:50

Geography, 08.07.2019 19:50