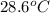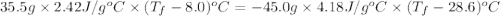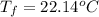Chemistry, 17.10.2019 06:00 steven2996
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (density = 1.0 g/ml) initially at 28.6 ∘c in an insulated beaker, what is the final temperature of the mixture, assuming that no heat is lost? (cetoh=2.42j/(g⋅∘

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (den...
Questions


Mathematics, 18.12.2020 08:10



Mathematics, 18.12.2020 08:10

Biology, 18.12.2020 08:10



Chemistry, 18.12.2020 08:10


Social Studies, 18.12.2020 08:10

Social Studies, 18.12.2020 08:10


Mathematics, 18.12.2020 08:10





Mathematics, 18.12.2020 08:10

Arts, 18.12.2020 08:10

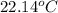
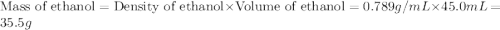
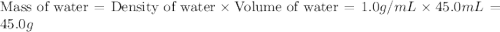
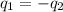
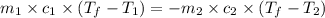
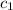 = specific heat of ethanol =
= specific heat of ethanol = 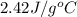
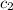 = specific heat of water =
= specific heat of water = 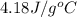
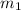 = mass of ethanol = 35.5 g
= mass of ethanol = 35.5 g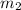 = mass of water = 45.0 g
= mass of water = 45.0 g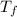 = final temperature of mixture = ?
= final temperature of mixture = ?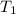 = initial temperature of ethanol =
= initial temperature of ethanol = 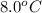
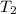 = initial temperature of water =
= initial temperature of water =