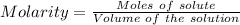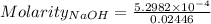Chemistry, 16.10.2019 01:30 jakails828
The concentration of a certain sodium hydroxide solution was determined by using the solution to titrate a sample of potassium hydrogen phthalate (abbreviated as khp). khp is an acid with one acidic hydrogen and a molar mass of 204.22 g/mol. in the titration, 24.46 ml of the sodium hydroxide solution was required to react with 0.1082 g khp. calculate the molarity of the sodium hydroxide.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 14:50
Write an equation to show action of positive and negative catalyst
Answers: 1

Chemistry, 23.06.2019 17:10
Two changes are described below. a green banana turns yellow and ripens. a layer of rust forms on an iron nail. which statement is true about the two changes? a) both are chemical changes because new substances are formed. b) both are physical changes because only the physical state of the substances change. c) a is a physical change due to a change of state, but b is a chemical change because new molecules are formed. d) a is a chemical change due to a change of state, but b is a physical change because new molecules are formed.
Answers: 1

Chemistry, 23.06.2019 19:30
If the standard reduction potential of a half-cell is positive, which redox reaction is spontaneous when paired with a hydrogen electrode? reduction both reduction and oxidation neither reduction nor oxidation oxidation
Answers: 1
You know the right answer?
The concentration of a certain sodium hydroxide solution was determined by using the solution to tit...
Questions

Mathematics, 25.02.2021 20:30

Biology, 25.02.2021 20:30


Mathematics, 25.02.2021 20:30


Mathematics, 25.02.2021 20:30


Mathematics, 25.02.2021 20:30


Mathematics, 25.02.2021 20:30




Business, 25.02.2021 20:30

Biology, 25.02.2021 20:30



History, 25.02.2021 20:30


Mathematics, 25.02.2021 20:30

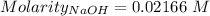
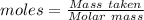
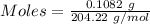
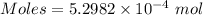
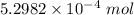 of KHP reacts with
of KHP reacts with