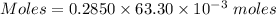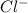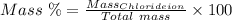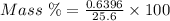Chemistry, 16.10.2019 00:00 Greghairston4839
The mass percent of cl⁻ in a seawater sample is determined by titrating 25.00 ml of seawater with agno₃ solution, causing a precipitation reaction. an indicator is used to detect the end point, which occurs when free ag⁺ ion is present in solution after all the cl⁻ has reacted. if 63.30 ml of 0.2850 m agno₃ is required to reach the end point, what is the mass percent of cl⁻ in the seawater (d of seawater = 1.024 g/ml)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


You know the right answer?
The mass percent of cl⁻ in a seawater sample is determined by titrating 25.00 ml of seawater with ag...
Questions


Computers and Technology, 03.09.2019 01:10





Mathematics, 03.09.2019 01:10


History, 03.09.2019 01:10

English, 03.09.2019 01:10




Mathematics, 03.09.2019 01:10


Mathematics, 03.09.2019 01:10

Physics, 03.09.2019 01:10



Social Studies, 03.09.2019 01:10

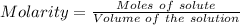
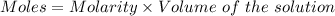
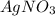 :
: