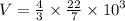Aparticular balloon can be stretched to a maximum surface area of 1257 cm2. the balloon is filled with 3.1 l of helium gas at a pressure of 754 torr and a temperature of 294 k . the balloon is then allowed to rise in the atmosphere. assume an atmospheric temperature of 273 k and determine at what pressure the balloon will burst. (assume the balloon to be in the shape of a sphere.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3


Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
Aparticular balloon can be stretched to a maximum surface area of 1257 cm2. the balloon is filled wi...
Questions


History, 22.06.2019 17:00









Biology, 22.06.2019 17:00


Geography, 22.06.2019 17:00

Chemistry, 22.06.2019 17:00



Mathematics, 22.06.2019 17:00

Computers and Technology, 22.06.2019 17:00

Physics, 22.06.2019 17:00