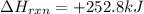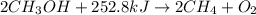When the enthalpy of the reaction is provided, it is always in the context of moles; i. e., δhrxn=+252.8 kj when two moles of ch3oh (methanol) decomposes into two moles of ch4 and one mole of o2. therefore, you can state that 252.8 kj is transferred when 2 mol ch3oh decompose or when 1 mol o2 is formed (based on the coefficients of the balanced equation).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2


Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
When the enthalpy of the reaction is provided, it is always in the context of moles; i. e., δhrxn=+...
Questions

Chemistry, 13.04.2021 17:20

Mathematics, 13.04.2021 17:20







Mathematics, 13.04.2021 17:30



Mathematics, 13.04.2021 17:30


Mathematics, 13.04.2021 17:30


Mathematics, 13.04.2021 17:30

Computers and Technology, 13.04.2021 17:30



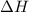 comes out to be positive and is written on the reactants side.Exothermic reactions: They are the reactions in which energy of reactants is more than the energy of the products. For these reactions, energy is released by the system. The
comes out to be positive and is written on the reactants side.Exothermic reactions: They are the reactions in which energy of reactants is more than the energy of the products. For these reactions, energy is released by the system. The