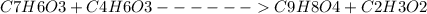Chemistry, 15.10.2019 18:00 kierafisher05
Aspirin can be prepared from salicylic acid ( c 7 h 6 o 3 ), which has a molar mass of 138.12 g/mol, and acetic anhydride ( c 4 h 6 o 3 ), which has a molar mass of 102.04 g/mol. the density of acetic anhydride is 1.082 g/ml. c 7 h 6 o 3 + c 4 h 6 o 3 ⟶ c 9 h 8 o 4 + c 2 h 3 o 2 what is the yield of aspirin ( c 9 h 8 o 4 ), which has a molar mass of 180.15 g/mol, possible when reacting 2.18 g of salicylic acid with 1.01 ml of acetic anhydride?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1


Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
Aspirin can be prepared from salicylic acid ( c 7 h 6 o 3 ), which has a molar mass of 138.12 g/mol,...
Questions



History, 27.02.2020 04:34


History, 27.02.2020 04:34


Social Studies, 27.02.2020 04:34




History, 27.02.2020 04:34

Mathematics, 27.02.2020 04:34

Mathematics, 27.02.2020 04:34



Social Studies, 27.02.2020 04:34

Computers and Technology, 27.02.2020 04:34