
The decomposition of n2o to n2 and o2 is a first-order reaction. at 730°c, the rate constant of the reaction is 1.94 × 10-4 min-1. if the initial pressure of n2o is 4.70 atm at 730°c, calculate the total gas pressure after one half-life. assume that the volume remains constant. slatter

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
The decomposition of n2o to n2 and o2 is a first-order reaction. at 730°c, the rate constant of the...
Questions






Mathematics, 12.11.2020 02:20


Mathematics, 12.11.2020 02:20

English, 12.11.2020 02:20

Engineering, 12.11.2020 02:20



English, 12.11.2020 02:20


Mathematics, 12.11.2020 02:20

Mathematics, 12.11.2020 02:20

Mathematics, 12.11.2020 02:20

Mathematics, 12.11.2020 02:20

Mathematics, 12.11.2020 02:20

Biology, 12.11.2020 02:20

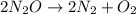
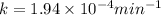
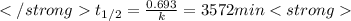
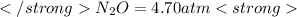
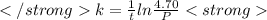
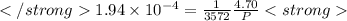
 after first half life = 2.35 = 4.70 - 2x
after first half life = 2.35 = 4.70 - 2x after first half life = 2x = 2(1.175) = 2.35 ATM
after first half life = 2x = 2(1.175) = 2.35 ATM

