
Chemistry, 15.10.2019 04:30 smmailloux2335
Consider a reactant with an order of 1: what effect does that reactant have on the rate equation when the concentration is doubled 1) the reaction rate is double with respect to that reactant 2) the order doesnt effect the reaction so there will be no change 3) the reaction rate quadruples with respect to that reactant. 4) the reaction rate isn't changed by individual reactants so there will be no change.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Consider a reactant with an order of 1: what effect does that reactant have on the rate equation wh...
Questions




Mathematics, 20.01.2021 21:10

Social Studies, 20.01.2021 21:10

Health, 20.01.2021 21:10

History, 20.01.2021 21:10

English, 20.01.2021 21:10


English, 20.01.2021 21:10



Mathematics, 20.01.2021 21:20

Mathematics, 20.01.2021 21:20

Mathematics, 20.01.2021 21:20


History, 20.01.2021 21:20

Mathematics, 20.01.2021 21:20

History, 20.01.2021 21:20

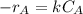
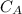 power is one (order 1), this could be proved just by checking it out in the rate law.
power is one (order 1), this could be proved just by checking it out in the rate law.

