Chemistry, 15.10.2019 01:00 BustD0wnAnt
1.24 grams of magnesium phosphate tribasic dissolved in 1 l of lemon juice. what is the ksp of the magnesium phosphate tribasic in lemon juice at room temperature?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 05:30
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1
You know the right answer?
1.24 grams of magnesium phosphate tribasic dissolved in 1 l of lemon juice. what is the ksp of the m...
Questions

Business, 19.02.2021 18:40


Mathematics, 19.02.2021 18:40

Chemistry, 19.02.2021 18:40

Physics, 19.02.2021 18:40







Mathematics, 19.02.2021 18:40





Mathematics, 19.02.2021 18:40


History, 19.02.2021 18:40

Business, 19.02.2021 18:40

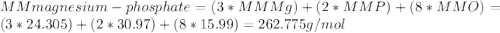
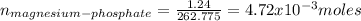
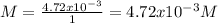
![K_{s} =[Mg^{2+}]^{3}[PO_{4}^{3-} ]^{2} =(3s)^{3} (2s)^{2}](/tpl/images/0320/1996/28dc2.png)