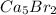Chemistry, 14.10.2019 20:20 breanna7667
Analysis of 20.0 g of a compound containing only calcium and bromine indicates that 4.00 g of calcium are present. what is the empirical formula of the compound formed?
a. ca7br2
b. ca3br2
c. cabr2
d. ca5br2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 23.06.2019 07:20
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3
You know the right answer?
Analysis of 20.0 g of a compound containing only calcium and bromine indicates that 4.00 g of calciu...
Questions

Mathematics, 10.11.2021 19:40

Mathematics, 10.11.2021 19:40




Mathematics, 10.11.2021 19:40





Social Studies, 10.11.2021 19:40

English, 10.11.2021 19:40

Mathematics, 10.11.2021 19:40

History, 10.11.2021 19:40


Mathematics, 10.11.2021 19:40

English, 10.11.2021 19:40

Mathematics, 10.11.2021 19:40

Mathematics, 10.11.2021 19:40

Mathematics, 10.11.2021 19:40