Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1


Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
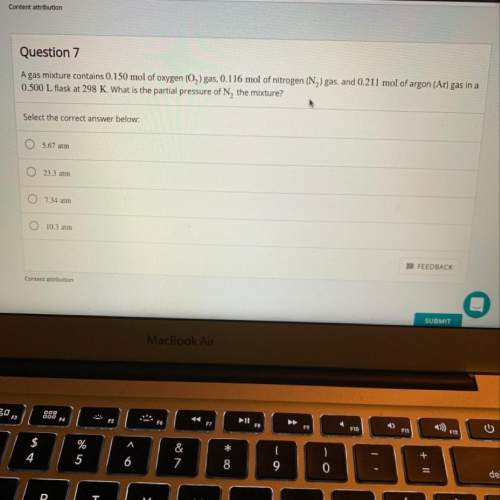
Agas mixture contains 0.150 mol of o2 gas, 0.116 mol of n2 gas, and 0.211 mol of ar gas in a 0.500 l...
Questions

Mathematics, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20

English, 26.01.2021 18:20


Mathematics, 26.01.2021 18:20





History, 26.01.2021 18:20




Mathematics, 26.01.2021 18:20

Biology, 26.01.2021 18:20

English, 26.01.2021 18:20

Mathematics, 26.01.2021 18:30

Biology, 26.01.2021 18:30