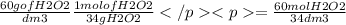Chemistry, 12.10.2019 19:30 kevonmajor
Hydrogen peroxide is sold commercially as an aqueous solution containing approximately 60 g/dm³ of hyrogen peroxide.
calculate the concentration in mol/dm³, of a solution containing 60.0 g/dm³ of hydrogen peroxide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1


Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Hydrogen peroxide is sold commercially as an aqueous solution containing approximately 60 g/dm³ of h...
Questions


Mathematics, 02.02.2021 18:30


Mathematics, 02.02.2021 18:30


English, 02.02.2021 18:30

Mathematics, 02.02.2021 18:30




Biology, 02.02.2021 18:40


Chemistry, 02.02.2021 18:40




Mathematics, 02.02.2021 18:40


Geography, 02.02.2021 18:40