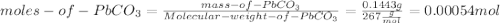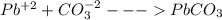The concentration of pb²⁺ in a sample of wastewater is to be determined by using gravimetric analysis. to a 100.0-ml sample of the wastewater is added an excess of sodium carbonate, forming the insoluble lead (ii) carbonate (267 g/mol) according to the balanced equation given below. the solid lead (ii) carbonate is dried, and its mass is measured to be 0.1443 g. what was the concentration of pb²⁺ in the original wastewater sample? pb²⁺(aq) + na₂co₃(aq) -> pbco₃(s) + 2na⁺(aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2


Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
The concentration of pb²⁺ in a sample of wastewater is to be determined by using gravimetric analysi...
Questions




Mathematics, 01.04.2020 05:22

Arts, 01.04.2020 05:22



Computers and Technology, 01.04.2020 05:22

Biology, 01.04.2020 05:22


Mathematics, 01.04.2020 05:23


English, 01.04.2020 05:23


Mathematics, 01.04.2020 05:23



History, 01.04.2020 05:23

Mathematics, 01.04.2020 05:36

Mathematics, 01.04.2020 05:36


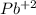 in the sample reacted with it. So we can say that the insoluble lead (II) carbonate
in the sample reacted with it. So we can say that the insoluble lead (II) carbonate 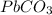 contains all the
contains all the