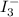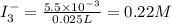The amount of i₃⁻(aq) in a solution can be determined by titration with a solution containing a known concentration of s₂o₂⁻³(aq) (thiosulfate ion). the determination is based on the net ionic equation 2s₂o₃²⁻(aq)+i₃⁻(aq)⟶s₄o₆²⁻(aq)+3i⁻( aq). given that it requires 38.1 ml of 0.440 m na₂s₂o₃(aq) to titrate a 25.0 ml sample of i₃⁻(aq), calculate the molarity of i₃⁻(aq) in the solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Given the table below, what is the chemical formula for a compound between rb and the nitrate ion no3 -1? nitrate ion no3 -1 phosphate po4 -3 sulfate so4 -2 acetate c2h3o2 -1 ammonium nh4 +1 chromate cro4 -2 carbonate co3 -2 dichromate cr2o7 -2 permanganate mno4 -1 sulfite so3 -2 rbno3 rb2no3 rb(no3)2 rb2(no3)3
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
The amount of i₃⁻(aq) in a solution can be determined by titration with a solution containing a know...
Questions

Biology, 15.12.2020 17:50



Chemistry, 15.12.2020 17:50


History, 15.12.2020 17:50

Mathematics, 15.12.2020 17:50

Chemistry, 15.12.2020 17:50


Mathematics, 15.12.2020 17:50

Mathematics, 15.12.2020 17:50

Mathematics, 15.12.2020 17:50

English, 15.12.2020 17:50

Mathematics, 15.12.2020 17:50


History, 15.12.2020 17:50


Mathematics, 15.12.2020 17:50

Social Studies, 15.12.2020 17:50

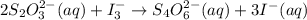
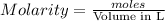
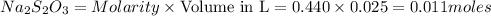
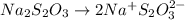
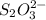 = 0.011
= 0.011 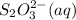 require 1 mole of
require 1 mole of 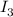
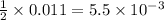 moles of
moles of