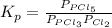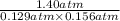Chemistry, 11.10.2019 23:30 sissygirl0807
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl₃(g)+cl₂(g)⇌pcl₅(g). a 7.5-l gas vessel is charged with a mixture of pcl₃(g) and cl₂(g), which is allowed to equilibrate at 450 k. at equilibrium the partial pressures of the three gases are ppcl₃ = 0.129 atm, pcl₂ = 0.156 atm, and ppcl₅ = 1.40 atm. what is the value of kp at this temperature? express your answer using three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

You know the right answer?
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl₃(g)+cl₂...
Questions






Mathematics, 18.12.2020 21:50


Mathematics, 18.12.2020 22:00


Mathematics, 18.12.2020 22:00

Chemistry, 18.12.2020 22:00


History, 18.12.2020 22:00


Mathematics, 18.12.2020 22:00

Biology, 18.12.2020 22:00


Mathematics, 18.12.2020 22:00


Mathematics, 18.12.2020 22:00

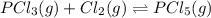
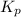 will be as follows.
will be as follows.