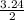Chemistry, 11.10.2019 23:30 redthangracing
2.00 mol of h2(g) and 1.00 mol of i2(g) are placed in a 1.00 l container, and they react to form hi(g). at equilibrium, it is found that 1.80 moles of hi(g) are present in the container. calculate k for the reaction: h2(g) i2(g) ⇄ 2 hi(g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 12:10
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1
You know the right answer?
2.00 mol of h2(g) and 1.00 mol of i2(g) are placed in a 1.00 l container, and they react to form hi(...
Questions

Mathematics, 04.05.2021 05:30


Mathematics, 04.05.2021 05:30


Mathematics, 04.05.2021 05:30




Advanced Placement (AP), 04.05.2021 05:30

Physics, 04.05.2021 05:30





Mathematics, 04.05.2021 05:30

Mathematics, 04.05.2021 05:30



Mathematics, 04.05.2021 05:30

![k = \frac{[[HI]^{2} }{[H2][I2]}](/tpl/images/0311/6358/eab06.png)
![k = \frac{[1.8]^{2} }{[2][1]}](/tpl/images/0311/6358/ea4d9.png)