
Chemistry, 11.10.2019 23:10 erniewernie
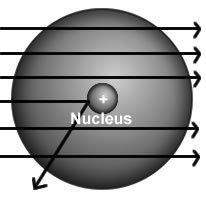
The results of rutherford's gold foil experiment gave him the evidence to arrive at two conclusions: (1) an atom was much more than just empty space and scattered electrons and (2)
a) in any atom, electrons orbit a central nucleus.
b) all atoms were composed of three subatomic particles.
c) an atom must have a positively charged center that contains most of its mass.
d) an atom must have an equal number of positive and negative particles in a central location.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1


Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 16:50
Question 2 (5 points) which of the following materials is useful for making molds because it has a low melting point? question 2 options: wood metal wax sand
Answers: 2
You know the right answer?
The results of rutherford's gold foil experiment gave him the evidence to arrive at two conclusions:...
Questions


Biology, 20.10.2019 15:50



History, 20.10.2019 15:50

Biology, 20.10.2019 15:50

Physics, 20.10.2019 15:50


Biology, 20.10.2019 15:50






Mathematics, 20.10.2019 15:50

Social Studies, 20.10.2019 15:50






