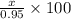Chemistry, 11.10.2019 22:30 kimsouther2
Amixture of kclo3 and kcl with a mass of 0.950 g was heated to produce o2. after heating, the mass of residue was 0.820 g. assuming all the kclo3 decomposed to kcl and o2, calculate the mass percent of kclo3 in the original mixture.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Amixture of kclo3 and kcl with a mass of 0.950 g was heated to produce o2. after heating, the mass o...
Questions

Mathematics, 01.04.2020 20:27

Social Studies, 01.04.2020 20:27






History, 01.04.2020 20:27

Computers and Technology, 01.04.2020 20:27

Law, 01.04.2020 20:27


Physics, 01.04.2020 20:27

Social Studies, 01.04.2020 20:27



History, 01.04.2020 20:27



Mathematics, 01.04.2020 20:28

Mathematics, 01.04.2020 20:28

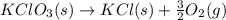
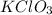 = 122.5 g/mol
= 122.5 g/mol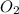 = 32 g/mole
= 32 g/mole
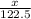
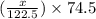
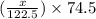 = 0.820 g
= 0.820 g