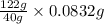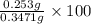An unknown amount of acid can often be determined by adding an excess of base and then back-titrating the excess. a 0.3471−g sample of a mixture of oxalic acid, which has two ionizable protons, and benzoic acid, which has one, is treated with 97.0 ml of 0.1090 m naoh. the excess naoh is titrated with 21.00 ml of 0.2060 m hcl. find the mass % of benzoic acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2


Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
An unknown amount of acid can often be determined by adding an excess of base and then back-titratin...
Questions


Mathematics, 11.05.2021 23:00






English, 11.05.2021 23:00


Social Studies, 11.05.2021 23:00

Mathematics, 11.05.2021 23:00





Mathematics, 11.05.2021 23:00


Mathematics, 11.05.2021 23:00

Chemistry, 11.05.2021 23:00

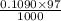
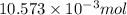
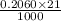
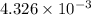 mol
mol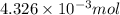
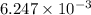 mol
mol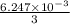
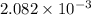 mol
mol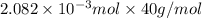
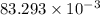 g
g