
Chemistry, 11.10.2019 03:00 Rainey1664
Naoh was titrated to the phenolphthalein endpoint with a known concentration of hcl. the student conducting the experiment placed 12.97 ml of 2.74 m hcl in a beaker with the indicator. before beginning the reaction, he noted that the buret read a volume of 4.50 ml. after reaching the endpoint, the buret read 13.65 ml. calculate the molarity of naoh that reacted upon reaching the endpoint. be sure to report your answer to the appropriate number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Naoh was titrated to the phenolphthalein endpoint with a known concentration of hcl. the student con...
Questions

World Languages, 25.08.2019 16:50

History, 25.08.2019 16:50


Mathematics, 25.08.2019 16:50

Mathematics, 25.08.2019 16:50



Mathematics, 25.08.2019 16:50


Advanced Placement (AP), 25.08.2019 16:50


History, 25.08.2019 16:50


English, 25.08.2019 16:50



Mathematics, 25.08.2019 16:50


Mathematics, 25.08.2019 16:50

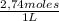 = 0,0347 moles HCl ≡ 0,0347 moles NaOH
= 0,0347 moles HCl ≡ 0,0347 moles NaOH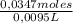 = 3,65M
= 3,65M

