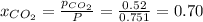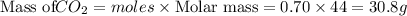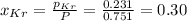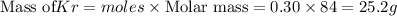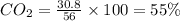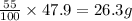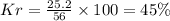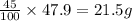Be sure to answer all parts. a mixture of co2 and kr weighs 47.9 g and exerts a pressure of 0.751 atm in its container. since kr is expensive, you wish to recover it from the mixture. after the co2 is completely removed by absorption with naoh(s), the pressure in the container is 0.231 atm. (a) how many grams of co2 were originally present? (b) how many grams of kr can you recover?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Be sure to answer all parts. a mixture of co2 and kr weighs 47.9 g and exerts a pressure of 0.751 at...
Questions

History, 17.11.2019 23:31

Mathematics, 17.11.2019 23:31

English, 17.11.2019 23:31

English, 17.11.2019 23:31

English, 17.11.2019 23:31

History, 17.11.2019 23:31


Law, 17.11.2019 23:31




Mathematics, 17.11.2019 23:31


Mathematics, 17.11.2019 23:31

English, 17.11.2019 23:31



Mathematics, 17.11.2019 23:31

Mathematics, 17.11.2019 23:31

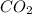 in mixture = 26.3 grams
in mixture = 26.3 grams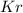 in mixture that can be recovered= 21.5 grams
in mixture that can be recovered= 21.5 grams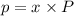
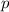 = partial pressure
= partial pressure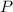 = total pressure = 0.751 atm
= total pressure = 0.751 atm = mole fraction
= mole fraction